March 24, 2025, marks a milestone for the medical device industry in Australia: on this date, the Unique Identification of Medical Devices (UDI) system officially came into effect, as set out in the Therapeutic Goods Legislation Amendment (Australian Unique Device Identification Database and Other Measures) Regulations 2025. With the introduction of the UDI, Australia strengthens its regulatory framework, bringing it in line with international standards.
This change is bound to have a major positive impact on the traceability, safety and post-marketing management of Medical Devices.
At this early stage, the UDI system for Medical Devices in Australia is available voluntarily. In other words, sponsors and manufacturers can already register and upload data to the national database, the Australian Unique Device Identification Database (AusUDID), managed by the Therapeutic Goods Administration (TGA).
Instead, the introduction of mandatory labelling requirements for Medical Devices will be gradual. The first deadline is set for July 1, 2026: from this date, sponsors and manufacturers who distribute most Medical Devices in Australia will be required to place the UDI on the device label and on all relevant packaging, as well as submit the data to AusUDID.
UDI Requirements
TGA amended the essential principles of Annex 1 of the Therapeutic Goods (Medical Devices) Regulations 2002 to integrate UDI requirements. Similar to other countries, the Australian UDI system provides:
- Assignment of a UDI code (UDI-DI and UDI-PI) for each medical device.
- Registration of UDI data in the AusUDID database;
- Inserting the UDI code on the label and packaging
- Direct Marking for some reusable devices.
In addition, two complementary formats are to be used:
- HRI (human-readable interpretation)
- AIDC (automatic data capture)
Thanks to this dual mode, traceability is ensured both manually and with automated systems throughout the supply chain.
Phased Implementation: Deadlines for Medical Devices
The compulsory UDI will follow a staggered schedule based on the risk classification of the product.
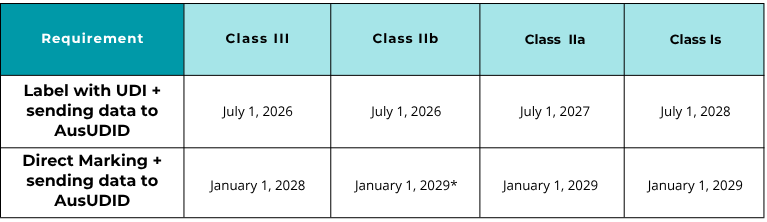
Direct marking for Class IIb devices may be subject to specific exceptions indicated by the TGA.
Sending UDI data to the TGA database
The introduction of the UDI system in Australia is part of a broader global harmonisation process involving more and more countries in the medical device industry. The aim is to make the entire life cycle of the device more effective and transparent, starting with its traceability, a key element for responsible and safe management. At the same time, the UDI system contributes to enhancing patient safety by facilitating rapid intervention in case of problems, such as recalls or safety alerts, and supporting more structured and timely post-marketing monitoring.
In this context, sponsors and manufacturers must start preparing from the very beginning. Establishing a phased compliance plan, updating internal procedures and becoming familiar with the operation of the AusUDID database are necessary steps to ensure a smooth transition and full compliance with Australian regulatory requirements.
>>> Thema offers support for the registration of Medical Devices in Australia, including UDI compliance. Thema consultants will guide you every step of the way, ensuring a smooth transition and compliance with Australian regulations.
SOURCE:
06/16/2025




